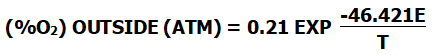Factory Price Furnace Oxygen Analyzer - Nernst N2032-O2/CO oxygen content and combustible gas two-component analyzer – Litong
Factory Price Furnace Oxygen Analyzer - Nernst N2032-O2/CO oxygen content and combustible gas two-component analyzer – Litong Detail:
Application range
The Nernst N2032-O2/CO oxygen content and combustible gas two-component analyzer is a comprehensive analyzer that can simultaneously detect oxygen content, carbon monoxide and combustion efficiency in the combustion process. It can monitor the oxygen content and carbon monoxide content in the flue gas during or after the combustion of boilers, furnaces, and kilns.
The analyzer mate with Nernst O2/CO probe can measure the oxygen content percentage O2% in the flue and furnace, the PPM value of carbon monoxide CO, the value of 12 combustible gases and the combustion efficiency of the combustion furnace in real time.
Application characteristics
After using Nernst N2032-O2/CO oxygen content and combustible gas two-component analyzer, users can save a lot of energy and control exhaust gas emissions.
The Nernst N2032-O2/CO oxygen content and combustible gas two-component analyzer is a unique technology that uses zirconia double-head structure developed after ten years of research and can simultaneously measure oxygen content and carbon monoxide content. It is currently a true in-line measurement technology.Low cost, high accuracy, can be measured online under various high moisture and high dust conditions.
In the process of peroxygen combustion, when the fuel gas and the combustion-supporting oxygen reach a certain dynamic equilibrium point, the carbon monoxide content will also change with the slight change in the amount of oxygen.The change trend of oxygen content and the change trend of carbon monoxide form the same superimposed trend.
Nernst O2/CO probe measuring principle
The Nernst O2/CO probe has dual electrodes, which can detect both the oxygen signal and the combustible signal at the same time.Because incomplete combustion flue gas contains carbon monoxide (CO), combustibles and hydrogen (H2).
The oxygen cell of the zirconia probe or oxygen sensor uses the oxygen potential generated by the different oxygen concentrations on the inside and outside of the zirconia at high temperature (greater than 650°C) to measure the oxygen content of the measured part.The outer part of the probe is made of stainless steel shell or alloy steel shell, which is composed of alloy steel heater, zirconia tube, thermocouple, wire, terminal board and box, see the schematic diagram.The zirconia tube of the probe is gas insulated from the inside and outside of the zirconia tube through a corresponding sealing device.
When the temperature of the zirconia probe head reaches 650°C or higher through the heater or the external temperature, the different oxygen concentrations on the inner and outer sides will generate corresponding electromotive force on the surface of the zirconia.The electric potential can be measured by the corresponding lead wire, and the temperature value of the part can be measured by the corresponding thermocouple.
When the oxygen concentration inside and outside the zirconia tube is known, the corresponding oxygen potential can be calculated according to the zirconia potential calculation formula.
The formula is as follows:
Where E is the oxygen potential, R is the gas constant, T is the absolute temperature value, PO2 INSIDE is the pressure value of the oxygen inside the zirconia tube, and PO2 OUTSIDE is the pressure value of the oxygen outside the zirconia tube.According to the formula, when the oxygen concentration inside and outside the zirconia tube is different, the corresponding oxygen potential will be generated.It can be known from the calculation formula that when the oxygen concentration inside and outside the zirconia tube is the same, the oxygen potential should be 0 millivolt (mV).
If the standard atmospheric pressure is one atmosphere and the oxygen concentration in the air is 21%, the formula can be simplified to:
()
When the oxygen potential is measured with a measuring instrument and the oxygen concentration inside or outside the zirconia tube is known, the oxygen content of the measured part can be obtained according to the corresponding formula.
The calculation formula is as follows: (At this time, the temperature in the zirconia part must be greater than 650°C )
(%O2) OUTSIDE (ATM) = 0.21 EXP T(-46.421E)
Characteristic curve

When the measured gas contains O2 and CO at the same time, due to the high temperature of the sensor and the catalytic effect of the platinum electrode area of the sensor, O2 and CO will react and reach a thermodynamic equilibrium state,the PO2 on the measured side has changed so that the oxygen partial pressure at equilibrium is P’O2.
This is because after the sensor is activated at high temperature, the process of O2 and CO reaction tending to balance is parallel to the process of O2 concentration diffusion. When the reaction reaches equilibrium, the diffusion of O2 concentration also tends to stabilize, so that the measured oxygen partial pressure at equilibrium is P’O2.
The following reactions occur in the negative area of the ZrO2 battery:
1/2 O2(PO2)+CO→CO2
When the reaction reaches equilibrium, the O2 concentration changes, PO2 is reduced to P’O2, and the conversion of gaseous oxygen molecules and O2 in the matrix is:
Negative electrode: O2 → 1/2 O2(P’O2)+2e
Positive electrode: 1/2 O2 (PO2)+2e → O2
The battery concentration difference process is: 1/2 O2 (PO2) → 1/2 O2(P’O2)
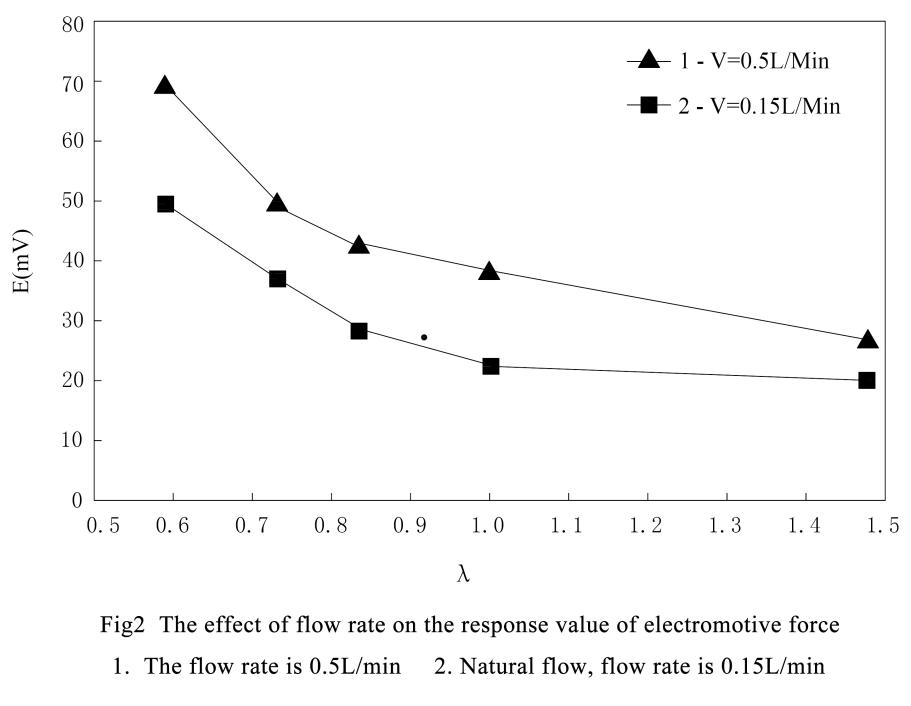
When the electromotive force of the sensor is compared with the number of moles of oxidation-reduction gas, the curve is a characteristic curve similar to a titration curve.
The shape of this characteristic curve under certain temperature, pressure and flow rate, the same sensor has exactly the same characteristic curve for the same kind of gas system.
Therefore, under an atmospheric pressure and the measured gas in natural flow, the comparison of the electromotive force and the number of moles of the O2-CO system by the zirconia sensor is a λ (λ=no2 /nco or volume percentage λ=O2 × V %/OCO × V %) characteristic curve.
When the Pt-Al2O3 catalyst is catalyzed at 600°C, the CO in the aerobic system can be completely converted to CO2, so the measured gas contains only oxygen after catalytic combustion.
At this time, the zirconia sensor measures the accurate oxygen content. Due to the relationship of the measured gas under the action of catalytic combustion, the CO content in the measured gas can be measured.The relationship between the reaction formula and the quantity before and after the catalytic combustion of the measured gas is as follows:
Suppose the concentration of carbon monoxide in the measured gas before catalysis is (CO), the concentration of oxygen is A1, and the concentration of oxygen in the measured gas after catalysis is A, then:
Before burning: (CO) A1
After burning: O A
Then: A=A1 – (CO)/2
And: λ =A1 /(CO)
So: A=λ × (CO)-(CO)/2
Result: (CO) = 2A /(2λ-1) (λ>0.5)
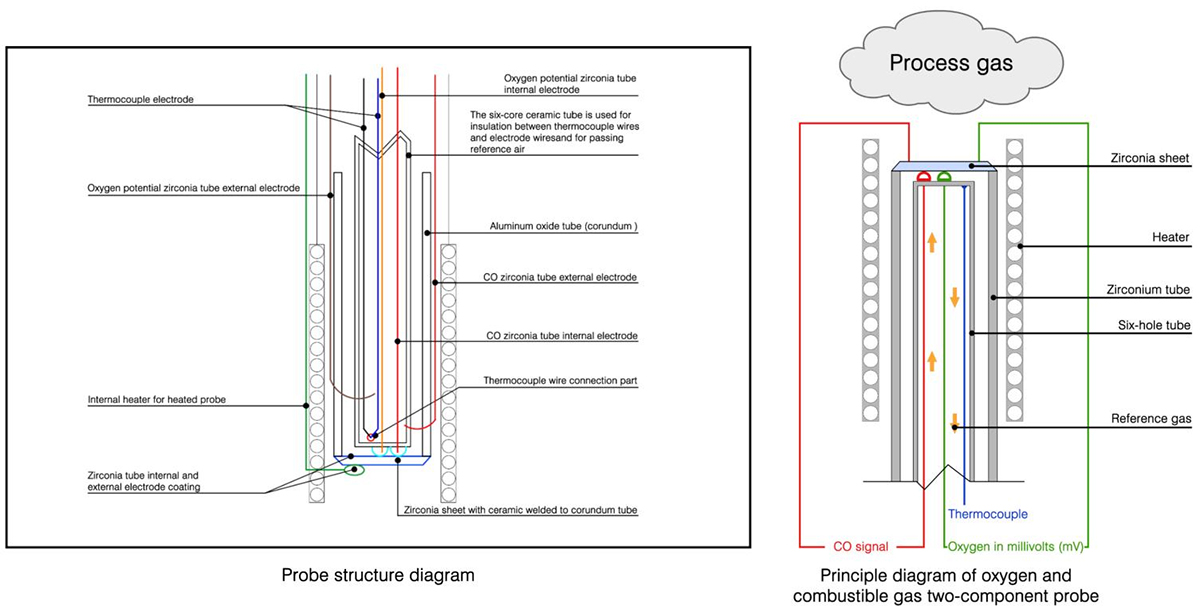
The structure principle of the O2/CO probe
The O2/CO probe has made corresponding changes on the basis of the original probe to realize the new combustion control function.In addition to detecting the oxygen content during the combustion process, the probe can also detect incompletely combusted combustibles (CO/H2), because carbon monoxide (CO) and hydrogen (H2) coexist in the flue gas of incomplete combustion.
The probe is the basic element that uses the electrochemical principle after heating of zirconia to realize the measurement.
A. O2 electrode (platinum)
B. COe electrode (platinum/precious metal)
C. Control electrode (platinum)
The core component of the probe is the zirconia composite sheet welded on the corundum tube to form a sealed tube and exposed to the flue gas channel of the combustion system.The use of built-in electrodes can effectively prevent corrosion components from damaging the electrodes and increase the service life.
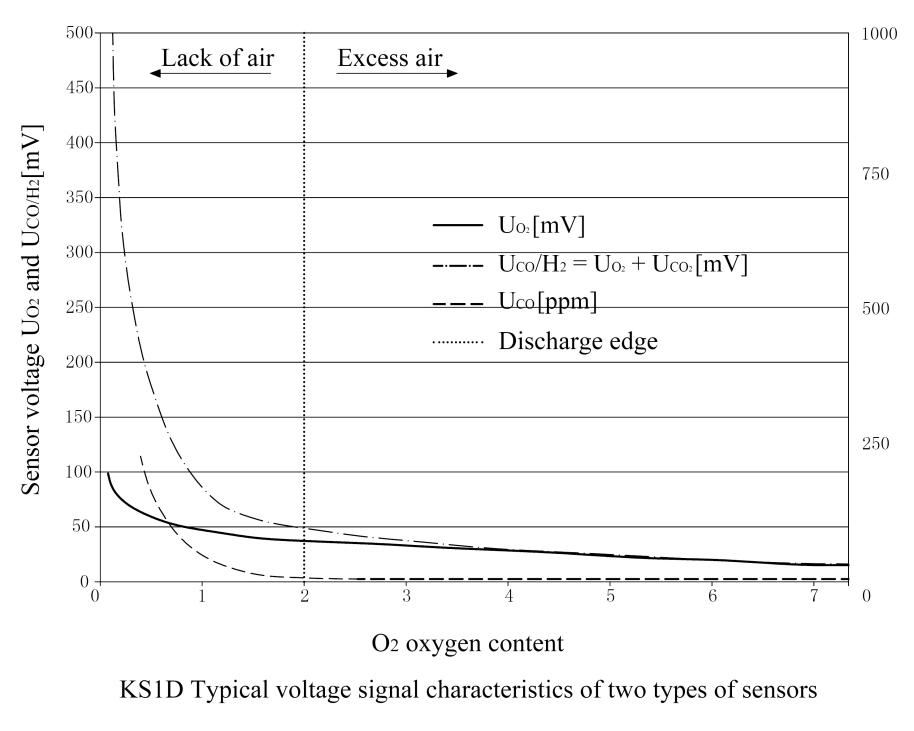
The functions of the COe electrode and the O2 electrode are the same, but the difference between the two electrodes is the electrochemical and catalytic properties of the raw materials, so that the combustible components in the flue gas such as CO and H2 can be identified and detected.In the state of complete combustion, the “Nernst” voltage UO2 is also formed at the COe electrode, and these two electrodes have the same curve characteristics. When detecting incomplete combustion or combustible components, the non-”Nernst” voltage UCOe will also be formed on the COe electrode, but the characteristic curves of the two electrodes move separately.(See typical graphs for both sensors)
The voltage signal UCO/H2 of the total sensor is the voltage signal measured by the COe electrode. This signal includes the following two signals:
UCO/H2 (total sensor) = UO2 (oxygen content) + UCO2/H2 (flammable components)
If the oxygen content measured by the O2 electrode is subtracted from the signal of the total sensor, the conclusion is:
UCOe (combustible component) = UCO/H2 (total sensor) - UO2 (oxygen content)
The above formula can be used to calculate the combustible component COe measured in ppm.The probe sensor is a typical voltage signal characteristic.The graph shows a typical curve (dashed line) of COe concentration when the oxygen content gradually decreases.
When combustion enters an area lacking air, at the so-called “emission edge” point, when insufficient air causes incomplete combustion, the corresponding COe concentration will increase significantly.
The obtained signal characteristics are shown in the probe curve diagram.
UO2 (continuous line) and UCO/H2 (dotted line).
When the air is surplus and the combustion is completely free of COe components, the sensor signal UO2 and UCO/H2 are the same, and according to the “Nernst” principle, the current oxygen content of the flue gas channel is displayed.
When approaching the “discharge edge”, the total sensor voltage signal UCO/H2 of the COe electrode increases at a disproportionate rate due to the additional non-Nernst COe signal.For the voltage signal characteristics of the sensor: UO2 and UCO/H2 relative to the oxygen content in the flue gas channel, the typical characteristics of the combustible component COe are also displayed here.
In addition to the voltage signals of the sensors UCO/H2 and UO2, the relatively dynamic sensor signals dU O2/dt and dUCO/H2 /dt and especially the fluctuation signal range of the COe electrode can be used to lock the “emission edge” of combustion.
(See “Incomplete combustion: the voltage fluctuation range of COe electrode UCO/H2“)
Technical characteristics
• Dual probe input function: One analyzer can be equipped with two probes, which can save the use cost and improve measurement reliability.
• Multiple output function: The analyzer has two 4-20mA current signal output and computer-computer communication interface RS232 or network interface RS485. One channel of oxygen signal output, the other channel of CO signal output.
• Measurement range: The oxygen measurement range is 10-30 to 100% oxygen content, and the carbon monoxide measurement range is 0-2000PPM.
• Alarm setting: The analyzer has 1 general alarm output and 3 programmable alarm outputs.
• Automatic calibration: The analyzer will automatically monitor various functional systems and automatically calibrate to ensure the accuracy of the analyzer during measurement.
• Intelligent system: The analyzer can complete the functions of various settings according to the predetermined settings.
• Display output function: The analyzer has a strong function of displaying various parameters and a strong output and control function of various parameters.
• Safety function: When the furnace is out of use, the user can control to turn off the heater of the probe to ensure safety during use.
• Installation is simple and easy: the installation of the analyzer is very simple and there is a special cable to connect with the zirconia probe.
Specifications
Inputs
• One or two zirconia probes or one zirconia probe + CO sensor
• Flue or spare thermometer type K, R, J, S type
• Pressure gas purge signal input
• Choice of two different fuels
• Explosion-proof safe operation control (only applicable to heated probe)
Outputs
Two linear 4~20mA DC signal output (maximum load 1000Ω)
• The first output range (optional)
Linear output 0~1% to 0~100% oxygen content
Logarithmic output 0.1~20% oxygen content
Micro-oxygen output 10-39 to 10-1 oxygen content
• The second output range (can be selected from the following)
Carbon monoxide content (CO) PPM value
Carbon dioxide (CO2)%
Combustible gas measurement PPM value
Combustion efficiency
Log oxygen value
Anoxic combustion value
Flue temperature
Secondary Parameter Display
• Carbon monoxide carbon (CO) PPM
• Combustible gas combustion efficiency
• Probe output voltage
• The temperature of the probe
• Ambient temperature
• Year month day
• Environment humidity
• Flue temperature
• Probe impedance
• Hypoxia index
• Operation and maintenance time
Computer/printer communication
The analyzer has an RS232 or RS485 serial output port, which can be directly connected to a computer terminal or a printer, and the probe and the instrument can be diagnosed through the computer.
Dust cleaning and standard gas calibration
The analyzer has 1 channel for dust removal and 1 channel for standard gas calibration or 2 channels for standard gas calibration output relays, and a solenoid valve switch that can be operated automatically or manually.
AccuracyP
± 1% of the actual oxygen reading with a repeatability of 0.5%. For example, at 2% oxygen the accuracy would be ±0.02% oxygen.
AlarmsP
The analyzer has 4 general alarms with 14 different functions, and 3 programmable alarms. It can be used for warning signals such as high and low oxygen content, high and low CO, and probe errors and measurement errors.
Display rangeP
Automatically display 10-30~100% O2 oxygen content and 0ppm~2000ppm CO carbon monoxide content.
Reference gasP
Air supply by micro-motor vibration pump.
Power Ruireqements
85VAC to 264VAC 3A
Operating Temperature
Operating Temperature -25°C to 55°C
Relative Humidity 5% to 95% (non-condensing)
Degree of Protection
IP65
IP54 with internal reference air pump
Dimensions and Weight
300mm W x 180mm H x 100mm D 3kg
Product detail pictures:

Related Product Guide:
Having a sound small business credit score, outstanding after-sales services and modern manufacturing facilities, we've got earned an fantastic reputation among our buyers across the globe for Factory Price Furnace Oxygen Analyzer - Nernst N2032-O2/CO oxygen content and combustible gas two-component analyzer – Litong, The product will supply to all over the world, such as: Suriname, Hanover, Swiss, Satisfaction and good credit to every customer is our priority. We focus on every detail of order processing for customers till they have received safe and sound products with good logistics service and economical cost. Depending on this, our products are sold very well in the countries in Africa, the Mid-East and Southeast Asia.
This company has the idea of "better quality, lower processing costs, prices are more reasonable", so they have competitive product quality and price, that's the main reason we chose to cooperate.